I do not have access to the RSC Veeva Vault for a specific study. Who should I contact?
Requests for access to a specific study in the RSC Veeva Vault system must be sent to the DAIDS TMF Team (NIAIDDAIDSTMF@mail.nih.gov) for approval. At minimum, your request must include your:
- Name
- Email address
- Study role (e.g., DAIDS TRP/PSP MO, OCSO PO/COR/MOB, or delegate uploading on behalf of an MO etc.,)
- List of protocol specific TMFs that you will need to access.
- Purpose/reason for needing access to the system
After completion of the RSC Veeva Vault system training, when should I expect access to the RSC Veeva Vault?
Access will be provided within 3-5 business days after completion of training.
To keep my RSC Veeva Vault access active how often do I log in?
It is recommended that users log in once a month to keep user access active. If you do not log in within 90 days, access is deactivated.
Should I log in to each study in the RSC Veeva Vault to keep my access active?
No, you don’t need to log into each TMF study to keep them active. If your Veeva Vault system level access is active, so is your assigned study access.
After I log in to the RSC Veeva Vault, is there any guidance on how to browse in the system?
The following documents, located on the RSC Website, will help you with navigating the RSC Veeva Vault system.
- DAIDS RSC Veeva Vault eTMF System Training - Video
- DAIDS RSC Veeva Vault eTMF System User Manual – PDF
- DAIDS RSC Quick Reference Card: Veeva Vault eTMF System – PDF
Note: If you are not already logged in to the DAIDS RSC Veeva Vault system, these links will take you to the Veeva Vault log in screen. Once you log in with your RSC Veeva Vault username and password, you will be routed to the document.
How do I view documents in the RSC Veeva Vault?
Please refer to document ‘DAIDS RSC eTMF Veeva Vault (VV) System User Manual - PDF’ which is located on the RSC Website.
How do I download a document from the RSC Veeva Vault?
Please refer to document ‘DAIDS RSC eTMF Veeva Vault (VV) System User Manual - PDF’ which is located on the RSC Website.
How do I upload/submit a document into the RSC Veeva Vault?
Please refer to document ‘DAIDS RSC eTMF Veeva Vault (VV) System User Manual - PDF’ which is located on the RSC Website.
Do I need a Submission Form for every time I make a submission to the RSC Veeva Vault?
Effective April 1, 2024, a TMF Submission Form is not required for TMF documents uploaded to the RSC Veeva Vault for any study.
How do I respond to any task that is assigned to me in the RSC Veeva Vault?
Please refer to document ‘DAIDS RSC eTMF Veeva Vault (VV) System Quick Reference Card v2 dated 2024-07-15 - PDF’ which is located on the RSC Website.
Is there a task called Audit Trail?
No, there is no such task. The Veeva Vault system provides a robust audit trail of all actions performed on a document or object record.
Why do I see both a “Version Number” and “Document Version Number” for certain documents I upload to the RSC Veeva Vault?
The “Version Number” metadata field refers to the version of the document within the system rather than to the Document Version Number itself. For example, when the document is in its initial review and approval workflow, the version number will be (0.1), and when it is in its final approved state, the version number will be (1.0). This is a noneditable field since it is system generated.
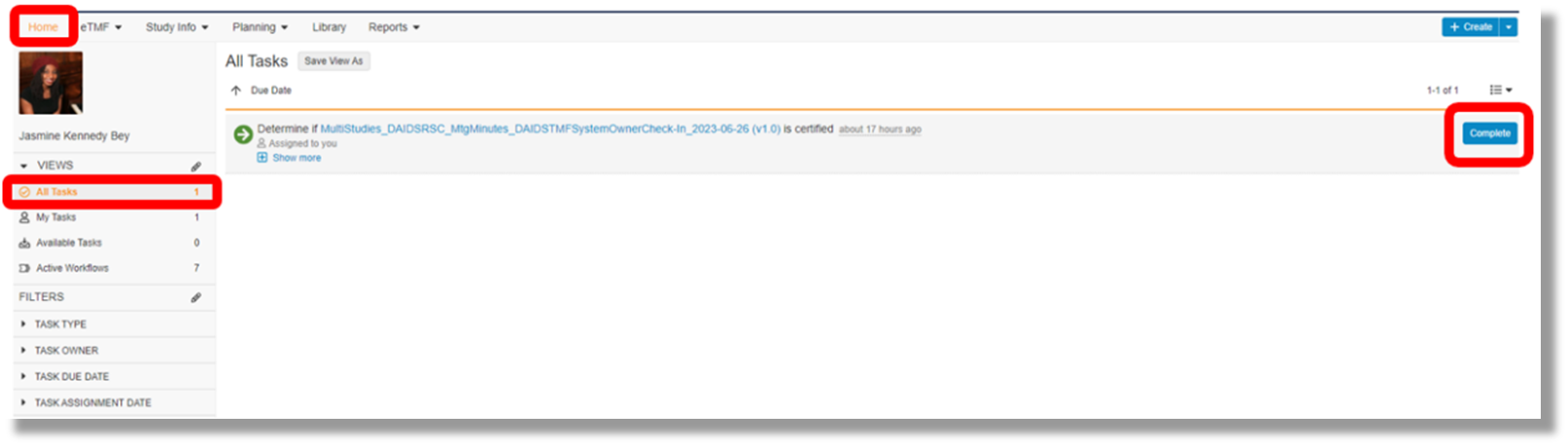
How do I complete a certification task?
A certification task will appear on your home page when you log into the RSC Veeva Vault. You must navigate to the task through one of 2 methods:
- Navigate to the Home Page ⇒ Find the task pending completion in the All Tasks View ⇒ Select “Complete”
- Navigate to the Document ⇒ Find the task pending completion and select “Complete.”
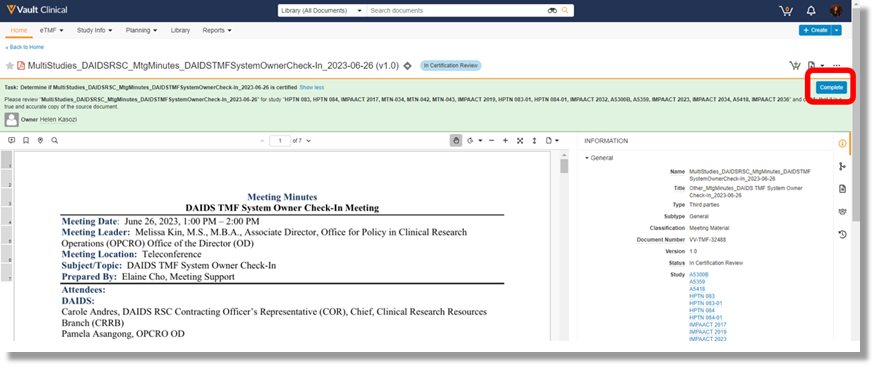
- Review the submitted document(s) to confirm that it is a certified copy and complete the required prompts.
I received a notification that a document I recently uploaded is a duplicate in the RSC Veeva Vault and I cannot see the duplicate in the system. How do I confirm that the document is a duplicate?
When any user begins to upload a TMF document that is already present in the RSC Veeva Vault, the system will flag that document and an alert message will appear stating the document is a duplicate. This alert will appear regardless of the user’s role. Cancel the submission and reach out to the RSC at DAIDS_TMF@tech-res.com to inquire about the next steps.
What if I have already submitted a document for a protocol and that document will also need to be filed in the eTMF for another study. Should I resubmit that document?
No, you don’t need to resubmit that document. You may reach out the DAIDS RSC TMF team to have them link this document already filed in the system to associate it to another study. This can be done via the system using the send as link method (below) or via email to the RSC (DAIDS_TMF@tech-res.com) by providing a document ID of the document that is already filed in the eTMF.
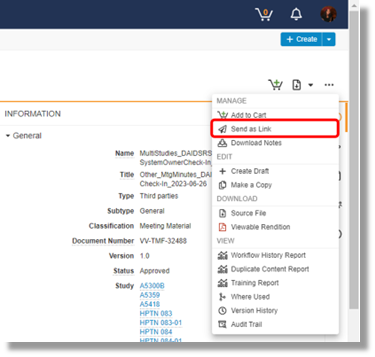
|
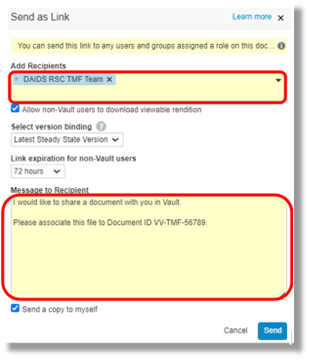
|
Note: To simplify the submission of documents that may apply to multiple protocols for future TMF’s, DAIDS PCs may maintain a list of documents that apply to multiple protocols. As new TMFs are started, that list can be reviewed, updated as needed, and those documents can be associated to the new TMFs.
